There Are 118 Known Elements
Fact of the day: There are 118 known elements.
Atoms make up most of the things around you. They make up every single substance.
Atoms are made up of three main types of particles (and their antimatter twins in anti atoms), the proton, the neutron, and the electron. I will cover these particles in greater detail in my next few posts.
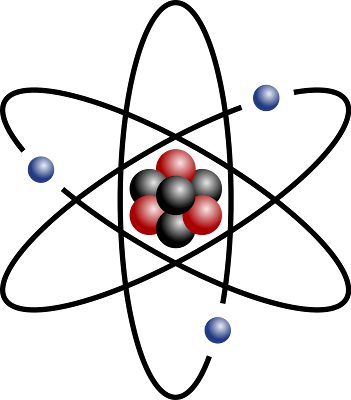
(above) A computerised image of a Bohr model atom.
Atoms combine to form molecules, via either ionic bonding (when one atom gives an electron to another atom, and then the atoms become charged, and then attract each other), covalent bonding (when atoms share electrons), and metallic bonding (when atoms with excess electrons shed them to create a lattice of positively charged atoms, and un-binded electrons).
Each atoms have an identity, which is determined by the number of protons in the nucleus of an atom. For example, every atom with 6 protons in its nucleus is carbon and every atom with 116 protons in its nucleus is livermorium. There are 118 known elements (atoms with the same number of protons in them) and are arranged into the Periodic Table Of Elements.
Atoms with the same number of protons in it nucleus, but with a different number of neutrons, are called isotopes. For example, Uranium-238 has 146 neutrons and 92 protons, and Uranium-235 has 143 neutrons and 92 protons.
The identity of an atom can change isotope or element via radioactive decay.
Most of an atom is empty space. 99.99999999999999% of a hydrogen atom is empty space. If this is put into proportion, and the atom is the size of the earth, the nucleus would have a diameter the length of Manhattan.
That means that everything is mostly empty space.
Atoms make up most of the things around you. They make up every single substance.
Atoms are made up of three main types of particles (and their antimatter twins in anti atoms), the proton, the neutron, and the electron. I will cover these particles in greater detail in my next few posts.
(above) A computerised image of a Bohr model atom.
Atoms combine to form molecules, via either ionic bonding (when one atom gives an electron to another atom, and then the atoms become charged, and then attract each other), covalent bonding (when atoms share electrons), and metallic bonding (when atoms with excess electrons shed them to create a lattice of positively charged atoms, and un-binded electrons).
Each atoms have an identity, which is determined by the number of protons in the nucleus of an atom. For example, every atom with 6 protons in its nucleus is carbon and every atom with 116 protons in its nucleus is livermorium. There are 118 known elements (atoms with the same number of protons in them) and are arranged into the Periodic Table Of Elements.
Atoms with the same number of protons in it nucleus, but with a different number of neutrons, are called isotopes. For example, Uranium-238 has 146 neutrons and 92 protons, and Uranium-235 has 143 neutrons and 92 protons.
The identity of an atom can change isotope or element via radioactive decay.
Most of an atom is empty space. 99.99999999999999% of a hydrogen atom is empty space. If this is put into proportion, and the atom is the size of the earth, the nucleus would have a diameter the length of Manhattan.
That means that everything is mostly empty space.



Comments
Post a Comment